Re-examination of complexation behaviors of V(v) and V(iv): experimental investigation and theoretical simulation
Abstract
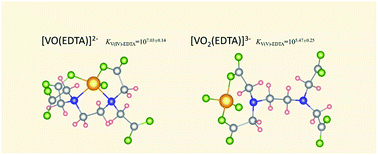
Vanadium(V) valence analysis is of great significance to the vanadium recovery and environmental safety of the vanadium extraction industry. The stability constants and stereo structures of complexes between EDTA and vanadium ions with various valences are only roughly estimated, which hinders the development of vanadium valence analysis. This work investigates in detail the complexation behavior of V(IV) and V(V), the most common valences in aqueous solution, with EDTA. The stability constants of [VO(EDTA)]2− and [VO2(EDTA)]3− complexes are determined to be 107.03±0.14 and 105.47±0.25 at pH 6, respectively. First-principles calculations reveal that the stereo structure of the [VO(EDTA)]2− complex is comprised of three 5-membered rings while that of the [VO2(EDTA)]3− complex is comprised of one 8-membered ring, which calls for the reconsideration of V–EDTA structures assumed commonly. This work has corrected the assumed stability constants and stereo structures of V–EDTA complexes and provided the essential knowledge to develop vanadium valence analysis in the vanadium industry.


 Please wait while we load your content...
Please wait while we load your content...