One-pot compositional and structural regeneration of degraded LiCoO2 for directly reusing it as a high-performance lithium-ion battery cathode†
Abstract
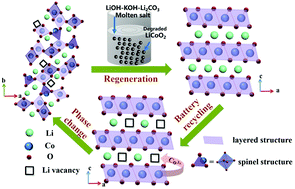
Recycling spent cathodes from Li-ion batteries (LIBs) is an appealing route to address environmental issues and resource shortage, but is plagued by effective and simple recycling techniques. Current recycling technologies generally involve multi-steps such as acid leaching, precipitation, smelting, or solid-state sintering, and obtained products possess inferior electrochemical properties. The current work explores a new regeneration method for failed LiCoO2 cathode material. The electrochemical performance of LiCoO2 is fully recovered through a single thermal-chemical treatment of spent LiCoO2 in molten LiOH–KOH–Li2CO3 under air atmosphere. The molten salt mixture presents suitable dissolving capacity, homogeneous thermal circumstance, abundant Li+, and high ion diffusion rate to decompose impurities, compensate for Li+ deficiency, and repair damaged structure through a “dissolution-recrystallization” mechanism. Under the optimized reaction temperature of 500 °C, the regenerated LiCoO2 possessed similar stoichiometric composition and crystalline structure of commercial LiCoO2. Importantly, the discharge capacity of spent LiCoO2 was recovered from 68.3 mA h g−1 to 144.5 mA h g−1, reaching the original level of commercial LiCoO2. Besides, the regenerated LiCoO2 also delivered superior cycling of 92.5% capacity retention after 200 cycles and excellent rate performance. This work presents an effective and extendable approach to regenerate spent cathode materials to retrieve their electrochemical performance.


 Please wait while we load your content...
Please wait while we load your content...