A case study targeting K fertilizer chemical synthesis with complete valorization of extraction by-products as an option†
Abstract
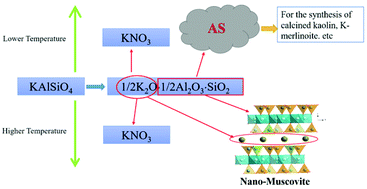
Here, we demonstrate processing techniques for KNO3 preparation through green chemical reaction engineering of abundant rock-forming minerals to achieve materials sustainability. In this study, chemical reactions at both low and high temperatures were conducted to investigate the extraction of potassium from kalsilite, KAlSiO4 for use as a K-fertilizer material and simultaneous transformation of kalsilite to materials such as aluminosilicate gel at low temperatures or muscovite at high temperatures; both these materials have many uses. In the low temperature range of 30 to 80 °C, the optimal conditions to extract potassium from KAlSiO4 and to obtain aluminosilicate gel were found to be as follows: by using the n(HNO3)/n(KAlSiO4) ratio of 1.1, solid to water (S/L) ratio of 1 : 10, a reaction time of 3 h and a temperature of 40 °C. In the high temperature range of 210 to 250 °C, both potassium extraction and crystallization of muscovite were achieved at 250 °C. The mechanisms of K extractions and the simultaneous crystallization of muscovite at higher temperatures were determined by using different characterization techniques such as X-ray diffraction, SEM/TEM, Fourier transform infrared spectroscopy (FTIR), and magic-angle-spinning nuclear magnetic resonance spectroscopy (MAS NMR). Results from the above characterization tools demonstrated that kalsilite can be converted to muscovite by dissolution and recrystallization under hydrothermal conditions at 250 °C while releasing K into solution simultaneously. Three comprehensive utilization routes of potassium-rich rocks for KNO3 preparation were proposed. Our current results suggest that molecular engineering of abundant natural minerals may lead to materials sustainability through a green chemical approach where reactions are conducted in a closed system to prevent pollution and recover all the components as resources.


 Please wait while we load your content...
Please wait while we load your content...