Separation of precious metals by split-anion extraction using water-saturated ionic liquids†
Abstract
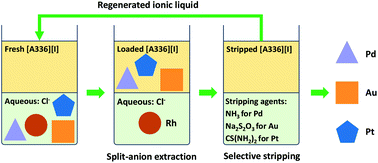
A split-anion solvent extraction process was developed for the separation of precious metal ions Au(III), Pt(IV), Pd(II) and Rh(III) from aqueous chloride media using water-saturated ionic liquids. The metal extraction and stripping behavior of the chloride form [A336][Cl], bromide form [A336][Br] and the iodide form [A336][I] of the quaternary ammonium ionic liquid Aliquat 336 were compared. The three ionic liquids extracted Au(III), Pd(II) and Pt(IV) quantitatively in most cases, whereas the co-extraction of Rh(III) was strongly dependent on the acidity and the chloride concentration. Among the studied ionic liquids, [A336][I] achieved the highest separation factors between Pd(II)/Rh(III), Pt(IV)/Rh(III), and Au(III)/Rh(III) at 6 mol L−1 Cl−. Additionally, the selective stripping of the individual metal ions Pd(II), Au(III), and Pt(IV) was only possible from loaded [A336][I] using ammonia solution (NH4OH), sodium thiosulfate (Na2S2O3), and thiourea ((NH2)2CS), respectively. A closed-loop flow sheet was designed for the recovery of the precious metals from chloride media using split-anion extraction with [A336][I]. The integrated process was demonstrated to be suitable for the purification of Rh(III), Pt(IV) and Pd(II) from a complex metal feed such as the leachate of spent automotive catalysts. The ionic liquid-based split-anion extraction process is simple, selective and effective for the sustainable separation of the precious metals, using only one green extractant [A336][I], which can be regenerated for consecutive extraction-stripping cycles.


 Please wait while we load your content...
Please wait while we load your content...