Sustainable oxidative cleavage of catechols for the synthesis of muconic acid and muconolactones including lignin upgrading†
Abstract
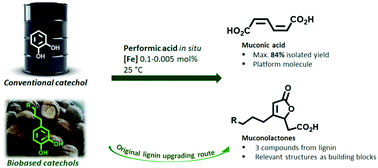
Muconic acid and muconolactones are attracting high interest as platform molecules for the synthesis of a variety of compounds, especially in the domain of materials. Although several technologies have been described for their synthesis, there is still a lack of performance, especially regarding green chemistry principles. In this study, we describe the development of an optimized catechol oxidative cleavage to muconic acid using performic acid in an intriguingly safe fashion. Common iron salts were used as catalysts to a level as low as 0.005 mol%, for a maximum turnover number of 13 200. Maximum muconic acid yield reached 84% after isolation by simple filtration. This procedure optimized on catechol was also efficient over a wide range of substituted catechols, providing access to muconolactones in a domino reaction. Noticeably, biobased catechols produced by a proven technology of lignin depolymerization were cleaved into muconolactones of high functional value. Applying this supplementary cleavage step to catechols obtained by lignin depolymerization was thus an ultimate way to maximize the economical value created from lignin. In contrast to other studies, lignin was not only depolymerized, but also depolymerization products were further transformed to take as much value from biomass as possible.


 Please wait while we load your content...
Please wait while we load your content...