Palladium-catalyzed ionic liquid-accelerated oxidative annulation of acetylenic oximes with unactivated long-chain enols†
Abstract
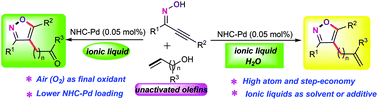
A palladium-catalyzed ionic liquid-accelerated oxidative cascade annulation/functionalization of acetylenic oximes with unactivated long-chain enols under aerobic conditions was accomplished. This newly developed protocol provides the rapid and straightforward synthetic strategy for the assembly of structurally diverse isoxazole architectures under mild conditions with high atom- and step-economy and exceptional functional group tolerance. Notably, the ionic liquid acts as not only a solvent in the reaction but also provides the excess halide ions to eliminate hydrochloride from acetylenic oximes. Moreover, this catalytic system could be recycled up to eight times and reused without significant loss of the catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...