Highly selective electrocatalytic oxidation of benzyl C–H using water as safe and sustainable oxygen source†
Abstract
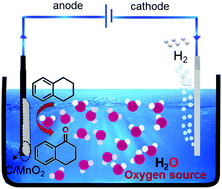
The selective oxidation of C–H bond is critical for feedstock manufacturing in chemical industry. Current strategies typically involve the use of oxygen or peroxide as the oxidation reagent under high temperature, which sets severe challenges in production sustainability and industrial safety. Herein, we demonstrate an environmental-friendly and safe electrocatalytic strategy for the selective oxidation of benzyl group to ketones at ambient conditions, while using water as the sole oxygen source. Water addition reduces the onset potential of anodic C–H oxidation, and produces 1-tetralone with satisfying conversion and excellent ketone to alcohol ratio. Layered MnO2 catalysts (with rich oxygen vacancies) further adjust the water affinity and facilitate the oxidation, leading to a significantly improved faradaic efficiency.


 Please wait while we load your content...
Please wait while we load your content...