Cell-free biocatalytic syntheses of l-pipecolic acid: a dual strategy approach and process intensification in flow†
Abstract
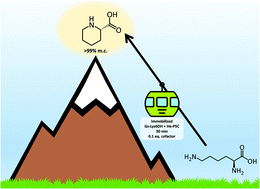
As an alternative to the traditional chemical synthesis or in vivo production of L-pipecolic acid, we have developed two ex vivo strategies using purified and immobilised enzymes for the production of this key building block. Firstly, a transaminase capable of lysine ε-deamination was coupled with a novel pyrroline-5-carboxylate reductase, yielding 60% conversion at the 50 mM scale with free enzymes and in situ recycling of the cofactor. A second, simpler, redox neutral system was then constructed by combining the pyrroline-5-carboxylate reductase with a lysine-6-dehydrogenase. This bienzymatic system, with catalytic amount of free cofactor yielded >99% of pipecolic acid in batch and, following co-immobilisation of both enzymes, it was applied as a packed-bed reactor in continuous flow achieving again a molar conversion of >99% with 30 min residence time, and a space–time yield up to 2.5 g L−1 h−1. The sustainability of the system was further improved by a catch-and-release strategy to purify the product, and recovery and recycling of the cofactor.
- This article is part of the themed collections: Welcoming our new Reaction Chemistry & Engineering Editorial Board members and 2020 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...