A paired electrolysis approach for recycling spent lithium iron phosphate batteries in an undivided molten salt cell†
Abstract
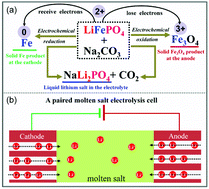
Recycling spent lithium-ion batteries can close the strategic metal cycle while reducing ecological and environmental footprints of the batteries. However, fewer efforts have been made for the recycling of spent LiFePO4 batteries owing to their relatively lower value than the batteries containing cobalt and nickel. In addition, excess acids are required to extract lithium from the olivine-structured LiFePO4. Herein, we report a paired electrolysis approach employing LiFePO4 as both the anode and the cathode, and molten carbonate as the electrolyte to reclaiming the retired LiFePO4 batteries. The paired electrolysis converts LiFePO4 to Fe at the cathode and to Fe3O4 at the anode while releasing Li+ and PO43− into the molten salt. In this scenario, the electric power drives the redox reactions and thereby separates Li, Fe, and PO43−. The recovery rate of Li reaches over 95.2%. Overall, the paired electrolysis cell containing dual LiFePO4 electrodes employs electrons to break down the olivine structure, which paves a green pathway to recycle various end-of-life LIBs with minimized secondary waste and energy consumption.


 Please wait while we load your content...
Please wait while we load your content...