Ultra-low temperature carbon (di)oxide hydrogenation catalyzed by hybrid ruthenium–nickel nanocatalysts: towards sustainable methane production†
Abstract
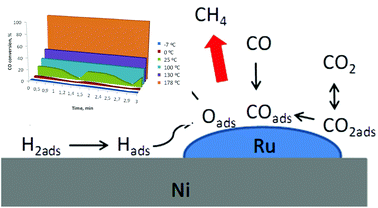
It is a paradox that the excess of carbon dioxide in our atmosphere can endanger lives and even the civilization that has been founded on carbon. Human addiction to carbon is persistent and therefore we need novel chemistry for the efficient conversion of CO2 to harmless or useful products. Accordingly, catalytic CO2 hydrogenation has been widely studied as a potential method for fuel engineering, and methane production in particular. Syngas, a blend of CO with H2 has been observed as an incomplete product of this reaction. Here we report a surprising discovery that syngas to methane conversion can be attained in flow at temperatures starting from −7 °C with a hybrid bimetallic Ru/Ni catalyst. In turn, the ultra-low temperature effect cannot be observed for the Re/Ni and Pd/Ni combinations. To our knowledge, this is the first report showing that such a process can be performed at a temperature lower than the freezing point of water. These ultra-low temperature conditions could potentially lead to sustainable methane production.


 Please wait while we load your content...
Please wait while we load your content...