Glucose oxidation to formic acid and methyl formate in perfect selectivity†
Abstract
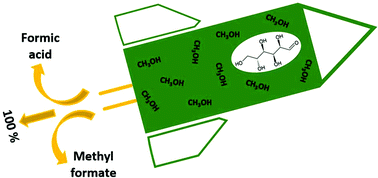
We report the highly remarkable discovery that glucose oxidation catalysed by polyoxometalates (POMs) in methanolic solution enables formation of formic acid and methyl formate in close to 100% combined selectivity, thus with only negligible sugar oxidation to CO2. In detail, we report oxidation of a methanolic glucose solution using H8[PV5Mo7O40] (HPA-5) as catalyst at 90 °C and 20 bar O2 pressure. Experiments with 13C-labelled glucose confirm unambiguously that glucose is the only source of the observed formic acid and methyl formate formation under the applied oxidation conditions. Our results demonstrate a very astonishing solvent effect for the POM-catalysed glucose oxidation. In comparison to earlier work, a step-change in product yield and selectivity is achieved by applying an alcoholic reaction medium. The extremely high combined yields of formic acid and methyl formate greatly facilitate product isolation as low-boiling methyl formate (bp = 32 °C) can simply be isolated from the reaction mixture by distillation.


 Please wait while we load your content...
Please wait while we load your content...