Acylation/cyclization of 1,6-dienes with ethers under catalyst- and base-free conditions†
Abstract
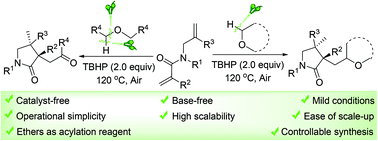
A concise and eco-friendly acylation/cyclization of 1,6-dienes with linear ethers under catalyst- and base-free conditions has been developed. tert-Butyl hydroperoxide (TBHP)-promoted tandem cleavage of C(sp3)–H and C(sp3)–O bonds of linear ethers is the key in this process, which provides a straightforward and efficient method to realize acylation reaction. The significant advantages of this method include catalyst- and base-free, mild reaction conditions, high scalability, operational simplicity, ease of scale-up, dual roles of ethers and controllable synthesis.


 Please wait while we load your content...
Please wait while we load your content...