Catalytic deoxygenation of bio-based 3-hydroxydecanoic acid to secondary alcohols and alkanes†
Abstract
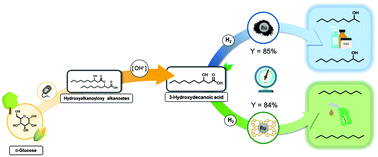
This work comprises the selective deoxygenation of bio-derivable 3-hydroxydecanoic acid to either linear alkanes or secondary alcohols in aqueous phase and H2-atmosphere over supported metal catalysts. Among the screened catalysts, Ru-based systems were identified to be most active. By tailoring the catalyst, the product selectivity could be directed to either secondary alcohols or linear alkanes. In the absence of a Brønsted acidic additive, 2-nonanol and 3-decanol were accessible with a yield of 79% and 6% respectively, both of which can be used in food and perfume industries as flavoring agents and fragrances. To produce alkanes, we successfully synthesized a bifunctional Ru/HZSM-5 catalyst. The acidic zeolite support facilitated the dehydration of the intermediary formed alcohols to alkenes, while the following hydrogenation occurred at the Ru centers. Thus, full 3-hydroxydecanoic acid deoxygenation to nonane and decane, which are both well-established as diesel and jet fuels, was achieved with up to 72% and 12% yield, respectively.


 Please wait while we load your content...
Please wait while we load your content...