Recycling of bonded NdFeB permanent magnets using ionic liquids†
Abstract
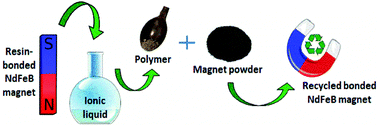
NdFeB permanent magnets are essential for modern day technology thanks to their excellent magnetic properties. Recycling of critical metals from sintered NdFeB permanent magnets has attracted a lot of attention over the last decade, whereas the recycling of (polymer or resin) bonded NdFeB magnets has been largely neglected. In this paper, an overview of the polymer or resin binders used in commercial bonded magnets is presented and different routes for the recycling of these magnets are explored. Three main types of polymers were found in commercial bonded NdFeB magnets: polyamides (PA6 and PA12), poly-p-phenylene sulfide (PPS) and epoxy. Both types of polyamide resins were easily dissolved by ionic liquids with coordinating anions (chloride, acetate or dialkylphosphate). Removal of the PPS resin was not possible by ionic liquid solvents, but only by using 1-chloronaphthalene and 1,3,5-triphenylbenzene at high temperatures. Although epoxy could be removed by several ionic liquids, reaction between the NdFeB powder and the ionic liquids was observed. A batch of PA6-bonded magnets was treated with an ionic liquid tributylethylphosphonium diethylphosphate, [P4442][Et2PO4], to selectively remove the polymeric portion. The PA-free magnet powder was found to retain >90% of its original magnetic properties. Two epoxy-bonded magnets produced with this recycled magnet powder showed magnetic properties that were close to those of commercial counterparts, proving the versatility of the process and that the materials loop could be successfully closed.


 Please wait while we load your content...
Please wait while we load your content...