One-step catalytic amination of naphthalene to naphthylamine with exceptional yield†
Abstract
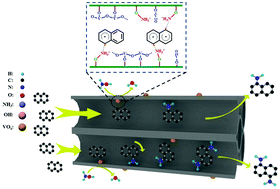
One-step amination of aromatic compounds to arylamines is a promising strategy with high atom economy and less environmental pollution. We propose for the first time a direct catalytic amination of naphthalene to naphthylamine and hydroxylamine using vanadium catalysts under mild conditions. Naphthylamine was obtained in 70% yield over the V2O5/HZSM-5 cayalyst in a one-step amination of naphthalene, which is higher than the yield obtained by state-of-the-art processes. The Brønsted acid sites, and the V–O–V and V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds of monovanadate in the V2O5/HZSM-5 catalyst are the active sites for the amination reaction and are responsible for the naphthalene activation and formation of NH3+ that acts as the active amination reagent. A possible reaction mechanism was also proposed by investigating real-time IR and in situ DRIFTS. This proposed one-step amination of naphthalene is superior to traditional nonselective nitration and hydrogenation processes, and some findings offer new insights to produce arylamines from aromatic compounds.
O bonds of monovanadate in the V2O5/HZSM-5 catalyst are the active sites for the amination reaction and are responsible for the naphthalene activation and formation of NH3+ that acts as the active amination reagent. A possible reaction mechanism was also proposed by investigating real-time IR and in situ DRIFTS. This proposed one-step amination of naphthalene is superior to traditional nonselective nitration and hydrogenation processes, and some findings offer new insights to produce arylamines from aromatic compounds.


 Please wait while we load your content...
Please wait while we load your content...