Electrochemical and direct C–H methylthiolation of electron-rich aromatics†
Abstract
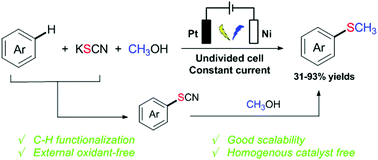
The electrochemical-induced C–H methylthiolation of electron-rich aromatics has been accomplished via a three component cross-coupling strategy. Potassium thiocyanate (KSCN) as both the supporting electrolyte and sulfur source and methanol as the methylation reagent are used. This protocol is versatile for various (hetero)aromatic compounds such as aniline, anisole and indole. The reaction proceeds under mild conditions without any metal catalyst, exogenous oxidant and highly toxic sulfur reagent. Importantly, such an electrochemical-induced methylthiolated reaction could be easily scaled up with good efficiency.


 Please wait while we load your content...
Please wait while we load your content...