Chemoselective O-formyl and O-acyl protection of alkanolamines, phenoxyethanols and alcohols catalyzed by nickel(ii) and copper(ii)-catalysts†
Abstract
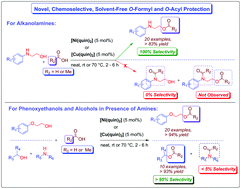
Achieving chemoselectivity is always crucial and challenging for bi-functional compounds, such as alkanolamines, that have both amines and alcohols as reactive functional groups. Achieving 100% selectivity for O-formyl and O-acyl protection of alkanolamines is one of the examples of such reactions. To avoid protection and deprotection steps and overcome this problem, a novel chemoselective, efficient, and simple protocol for functional group protection as O-formylation and O-acylation of alkanolamines and phenoxyethanols and competitive O-selectivity between alcohols and amines, catalyzed by Ni(II) and Cu(II) complexes with 8-hydroxyquinoline at a catalyst loading of only 5 mol% in a homogeneous medium has been presented here. Good to excellent yields are achieved in the absence of a solvent for O-formylation at room temperature with formic acid as the formyl source and O-acylation at 70 °C with acetic acid as the acyl source. In addition, minimal effluent and waste are generated during this reaction, as the corresponding sodium salts of acids could be recovered during the process and can be reused. This chemistry readily tolerates a variety of functional groups, as demonstrated by 20 examples with 100% chemoselectivity for O-formylation and O-acylation of alkanolamines and 30 examples of O-formylation and O-acylation of phenoxyethanols and alcohols in the presence of amines which have been synthesized successfully.


 Please wait while we load your content...
Please wait while we load your content...