A sustainable strategy for the assembly of Glypromate® and its structurally-related analogues by tandem sequential peptide coupling†
Abstract
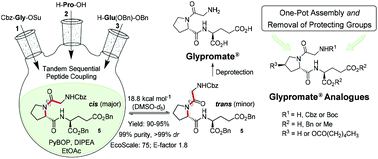
This work describes an improved and greener methodology of solution-phase synthesis for the preparation of Glypromate® (glycyl-L-prolyl-L-glutamic acid, GPE), a potent neuropeptide for applications in neurodegenerative conditions such as Huntington's, Parkinson's and Alzheimer's diseases. This protocol comprises the assembly of the perbenzylated form of Glypromate® [Cbz-Gly-Pro-Glu(OBn)-OBn (5)] from L-proline. Following a tandem sequential peptide coupling strategy, two chemoselective peptide bonds are formed without the need for purifying the intermediates ensuing a one-pot fashion synthesis. EcoScale score and E-factor were selected as the green metrics to assess the environmental impact of the preparation of tripeptide 5 using this protocol. After optimization and application of greener conditions, intermediate 5 was obtained with 95% global yield and 99% purity (NMR, HRMS, and rp-HPLC), with excellent final EcoScale score of 75 out of 100 and global E-factor of 1.8. Glypromate® is achieved by removing N- and C-protecting groups by hydrogenolysis using Pd/C as the catalyst in 98% yield, avoiding chromatographic techniques. Moreover, the protocol ensures stereochemical integrity (determined by VT-NMR and rp-HPLC) and was also successfully applied for the preparation of structurally-related Glypromate® analogues with higher degree of molecular complexity compatible with functionalized amino acids with different side chains. For the first time a one-pot protocol for the assembly of tripeptides with the removal of protecting groups in the same reaction vessel is reported.


 Please wait while we load your content...
Please wait while we load your content...