Using dialkyl amide via forming hydrophobic deep eutectic solvents to separate citric acid from fermentation broth†
Abstract
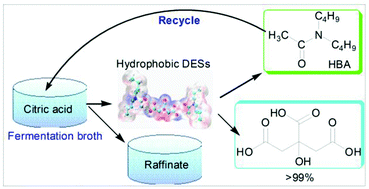
Nowadays, developing appropriate technology is one of the biggest challenges for society to reduce environmental impact. In this research, to avoid the traditional calcium salt method which produces a large amount of waste gypsum residue, a new way of separating citric acid from fermentation broth was developed by forming hydrophobic deep eutectic solvents (DESs), in which amide and citric acid were used as the hydrogen bond acceptor and donor respectively when amide was in contact with the fermentation broth containing citric acid. Among these amides, C10H21NO was found to be an efficient hydrogen bond acceptor forming hydrophobic DESs with the citric acid based on the molecular size and shape and has the largest hydrophobic equilibrium constant of 3.14. The hydrophobic DES formation mechanism was studied by analyzing the chemical bonds using FT-IR and quantum chemical (QC) calculations. C10H21NO was regenerated by elevating the temperature of the hydrophobic DESs. The regenerated C10H21NO exhibited good recycling properties with no obvious reduction of the ability to form hydrophobic DESs. This effective way of obtaining high-quality citric acids provides new ideas for the separation of other carboxylic acids.


 Please wait while we load your content...
Please wait while we load your content...