Selective conversion of lignin to ethylbenzene†
Abstract
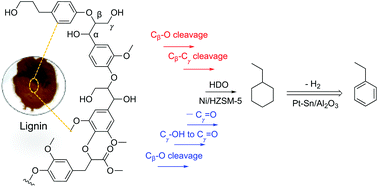
Lignin, an abundant renewable aromatic resource, has a complex structure composed of various C9 propyl phenol units with cross-linked C–C, ester and ether linkages. Herein, we report a two-step process for the selective production of ethylbenzene from corncob lignin valorization. This process starts with hydrodeoxygenation of lignin to C8 ethylcyclohexane (42%), C9–C17 cyclic alkanes (bio-jet fuel range, 38%), and some C3–C7 alkanes (gasoline range, 20%) over Ni/Silicalite-1 (Ni/S-1) at 300 °C and 6 MPa H2 using a non-polar solvent. After separation by distillation, the subsequent dehydrogenation of ethylcyclohexane over Pt–Sn/Al2O3 leads to the formation of ethylbenzene with 99.3% yield at 500 °C and 0.5 MPa H2 in a continuous flow reactor. It is demonstrated that the selective production of C8 ethylcyclohexane from lignin specifically proceeds by thermal cracking and hydrogenolysis on Ni nanoparticles (NPs). The thermal cracking of lignin eliminates the Cβ–Cγ bond in the side 3-C chain of the depolymerized C9 radicals, as formed by the homolytic cleavage of β-O-4 linkages in lignin via a quinone methide reaction pathway. Additionally, Ni NPs facilitate the decarboxylation of the carbonyl groups formed from the end hydroxyl and ester groups of C9 lignin units. The established strategy enables the selective production of value-added ethylbenzene from lignin valorization and the simultaneous formation of a qualified bicyclic alkane bio-jet fuel with high thermal stability.


 Please wait while we load your content...
Please wait while we load your content...