Selective conversion of chitin to levulinic acid catalyzed by ionic liquids: distinctive effect of N-acetyl groups†
Abstract
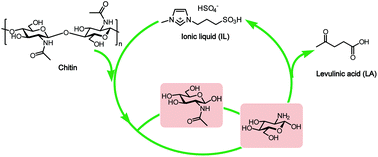
Selective and green conversion of chitin to levulinic acid (LA) has been realized by the catalysis of the ionic liquid (IL) 1-methyl-3-(3-sulfopropyl)imidazolium hydrogen sulfate ([C3SO3Hmim]HSO4) up to a yield of 67.0%. The relationship between the structure of IL and the yield of LA was investigated, demonstrating that both acidity and the hydrogen bonding ability of IL dictate its catalytic activity. Furthermore, by means of NMR, IR, SEM, GPC analyses and determination of the degree of acetylation (DA), two approaches of deacetylation–depolymerization to glucosamine (GlcN) and direct depolymerization to N-acetylglucosamine (GlcNAc) were proposed as chitin hydrolysis mechanisms, which occur only at the outer surface while leaving the interior crystalline chitin intact. The N-acetyl groups in chitin construct strong intramolecular and intermolecular hydrogen bond networks that contribute to the integration of the crystalline structure and building up of barriers to shield the accessibility of glycosidic linkages with the acidic catalyst.


 Please wait while we load your content...
Please wait while we load your content...