Hydrogen production from formic acid catalyzed by a phosphine free manganese complex: investigation and mechanistic insights†
Abstract
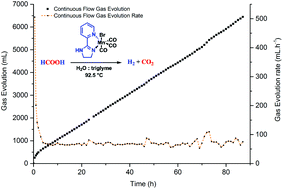
Formic acid dehydrogenation (FAD) is considered as a promising process in the context of hydrogen storage. Its low toxicity, availability and convenient handling make FA attractive as a potential hydrogen carrier. To date, most promising catalysts have been based on noble metals, such as ruthenium and iridium. Efficient non-noble metal systems like iron were designed but manganese remains relatively unexplored for this transformation. In this work, we present a panel of phosphine free manganese catalysts which showed activity and stability in formic acid dehydrogenation. The most promising results were obtained with Mn(pyridine-imidazoline)(CO)3Br yielding >14 l of the H2/CO2 mixture and proved to be stable for more than 3 days. Additionally, this study provides insights into the mechanism of formic acid dehydrogenation. Kinetic experiments, Kinetic Isotopic Effect (KIE), in situ observations, NMR labeling experiments and pH monitoring allow us to propose a catalytic cycle for this transformation.


 Please wait while we load your content...
Please wait while we load your content...