Vanillin inhibits PqsR-mediated virulence in Pseudomonas aeruginosa
Abstract
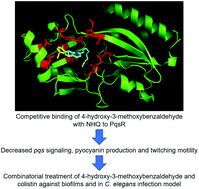
Reduced efficacy of antibiotics in bacterial diseases is a global concern in clinical settings. Development of anti-virulence compounds which disarm bacterial virulence is an attractive therapeutic agent for complementary antibiotics usage. One potential target for anti-virulence compounds is quorum sensing (QS), the intercellular communication system in most pathogens, such as Pseudomonas aeruginosa. QS inhibitors (QSIs) can inhibit QS effectively, attenuate QS-mediated virulence, and improve host clearance of infections. While studies focused on developing homoserine-based las QSI, few targeted the quinolone-based pqs QS, which implicated host cytotoxicity and biofilm formation. It is imperative to develop novel anti-pqs-QS therapeutics for combinatorial antibiotic treatment of microbial diseases. We employed a gfp-based transcriptional pqs biosensor to screen a natural compounds library and identify vanillin (4-hydroxy-3-methoxybenzaldehyde), the primary phenolic aldehyde of vanilla bean. The vanillin inhibited pqs expression and its associated phenotypes, namely pyocyanin production and twitching motility in P. aeruginosa. Molecular docking results revealed that vanillin binds to the active site of PqsR, the PQS-binding response regulator. Combinatorial treatment of vanillin with antimicrobial peptide (colistin) inhibited biofilm growth in vitro and improved treatment in the in vivo C. elegans acute infection model. We demonstrated that vanillin could dampen pqs QS and associated virulence, thus providing novel therapeutic strategies against P. aeruginosa infections.


 Please wait while we load your content...
Please wait while we load your content...