Anti-inflammatory action of two novel peptides derived from peanut worms (Sipunculus nudus) in lipopolysaccharide-induced RAW264.7 macrophages
Abstract
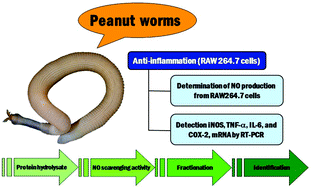
Peanut worm (Sipunculus nudus Linn.) protein was hydrolyzed by three proteases, and NO scavenging activity of the protein hydrolysates was evaluated. The hydrolysate obtained using Alcalase® showed the highest NO scavenging activity. This hydrolysate was fractionated using 10-, 5-, and 3 kDa molecular weight cut-off membranes, and the lowest MW fraction (<3 kDa) exhibited the highest NO scavenging activity. The <3 kDa fraction was further purified by gel filtration and high-performance liquid chromatographies. The peptides in the HPLC fraction with the strongest anti-NO activity were identified by quadrupole-time-of-flight mass spectrometry as LSPLLAAH (821.48 Da) and TVNLAYY (843.42 Da). Both peptides and the corresponding pure synthetic peptides inhibited NO production by RAW 264.7 macrophages without cytotoxicity. Quantitative real-time RT-PCR analysis showed that peptides LSPLLAAH and TVNLAYY reduced expression of proinflammatory cytokine genes iNOS, IL-6, TNF-α, and COX-2 in RAW 264.7 macrophages, suggesting that these peptides are novel anti-inflammatory candidates.


 Please wait while we load your content...
Please wait while we load your content...