Catalytic activity and mechanism of fluorinated MgO film supported on 3D nickel mesh for ozonation of gaseous toluene†
Abstract
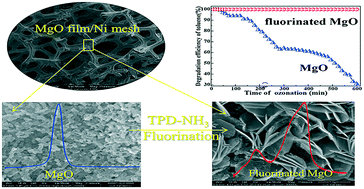
A nano-MgO film supported on 3D nickel mesh was prepared via an electrodeposition–thermolysis process. The film possessed high catalytic activity for the ozonation of toluene, but suffered from deactivation. Interestingly, after fluorination, its breakthrough time was 20 times longer (i.e., from 30 to 600 min) under 50% relative humidity. This significant enhancement was attributed to the formation of a fluorinated MgO film, Mg(OH)F, as confirmed using XRD, XPS, SEM, etc. Mg(OH)F possessed two typical acidic sites (Brønsted and Lewis sites), and they could co-catalyze O3 to generate more highly active ˙OH and O radicals. In particular, its Brønsted acidity could considerably decrease the accumulation of acidic intermediates on the film, suppressing its deactivation. Fluorinated MgO, Mg(OH)F, could be applied as a multifunctional ozonation catalyst for the degradation of other refractory pollutants.


 Please wait while we load your content...
Please wait while we load your content...