Modeling performance of rhamnolipid-coated engineered magnetite nanoparticles for U(vi) sorption and separation†
Abstract
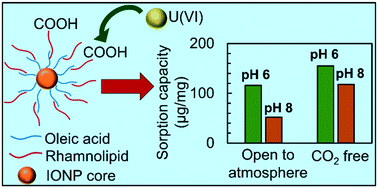
Based on tunable properties, engineered nanoparticles (NPs) hold significant promise for water treatment technologies. Motivated by concerns regarding toxicity and non-biodegradability of some nanoparticles, we explored engineered magnetite (Fe3O4) nanoparticles with a biocompatible coating. These were prepared with a coating of rhamnolipid, a biosurfactant primarily obtained from Pseudomonas aeruginosa. By optimizing synthesis and phase transfer conditions, particles were observed to be monodispersed and stable in water under environmentally relevant pH and ionic strength values. These materials were evaluated for U(VI) removal from water at varying dissolved inorganic carbon and pH conditions. The rhamnolipid-coated iron oxide nanoparticles (IONPs) showed high sorption capacities at pH 6 and pH 8 in both carbonate-free systems and systems in equilibrium with atmospheric CO2. Equilibrium sorption behavior was interpreted using surface complexation modeling (SCM). Two models (diffuse double layer and non-electrostatic) were evaluated for their ability to account for U(VI) binding to the carboxyl groups of the rhamnolipid coating as a function of the pH, total U(VI) loading, and dissolved inorganic carbon concentration. The diffuse double layer model provided the best simulation of the adsorption data and was sensitive to U(VI) loadings as it accounted for the change in the surface charge associated with U(VI) adsorption.


 Please wait while we load your content...
Please wait while we load your content...