The Dr Jekyll and Mr Hyde of lithium sulfur batteries
Abstract
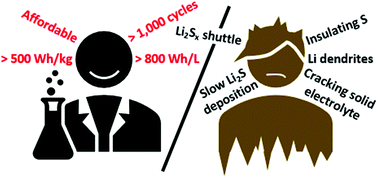
Although the concept of a lithium–sulfur (Li–S) battery promises an energy density surpassing that of conventional Li-ion cells, prototype cells have lagged far behind. As research on Li–S has progressed, four limiting challenges for the sulfur electrode have now emerged that must be addressed to facilitate their realization, including slow lithium polysulfide deposition kinetics at low electrolyte/sulfur ratios, the lithium polysulfide shuttle, the low electrical conductivity of sulfur active material, and the cracking of solid electrolytes due to the repeated expansion and contraction of sulfur active material. Notably, the challenges of both slow deposition kinetics and the lithium polysulfide shuttle only arise when using sulfur active material with the most common electrolyte solvents used in Li–S cells: liquid ethereal mixtures, such as DOL:DME. Consequently, a reckoning of ether-based electrolytes is at hand, which has shifted research focus toward solid electrolytes and alternative sulfur-based active materials; however, these alternative strategies have their own challenges. Meanwhile, dendrite growth and insufficient coulombic efficiency still plague lithium metal electrodes in both liquid and solid electrolytes at relevant current densities. Many approaches to mitigate dendrite growth have been attempted, including artificial SEIs, nucleation aids, high surface area current collectors and solid electrolytes. Despite successes, further work is needed to attain a sufficient level of performance for lithium metal to be used in commercial Li–S cells. Herein we discuss the strengths and weaknesses of many proposed solutions to these challenges with an eye toward the ever-present goal of competing with conventional Li-ion energy densities.


 Please wait while we load your content...
Please wait while we load your content...