Two derivatives of phenylpyridyl-fused boroles with contrasting electronic properties: decreasing and enhancing the electron accepting ability†
Abstract
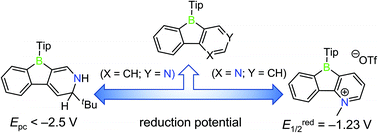
Two derivatives of phenylpyridyl-fused boroles were prepared via functionalization of the pyridyl groups, namely to an electron-rich dihydropyridine moiety (compound 1) and an electron-deficient N-methylpyridinium cation (compound 2). Due to strong conjugation between the dihydropyridine moiety and the boron atom, the reduction potential of compound 1 shifts cathodically. In contrast, compound 2 exhibits three reduction processes with a first reversible reduction potential anodically shifted in comparison to its precursor (TipPBB2) or the non-borylated framework 1-methyl-2-phenylpyridin-1-ium triflate (3). The significantly anodically shifted reduction potential indicates the extreme electron deficiency of compound 2, which also leads to the reversible coordination of THF. Photophysical properties of both compounds in different solvents were investigated. Theoretical studies further support the strong conjugation in the ground state of compound 1 and the electron-deficient property of compound 2.


 Please wait while we load your content...
Please wait while we load your content...