2-Pyridone-stabilized iridium silylene/silyl complexes: structure and QTAIM analysis†
Abstract
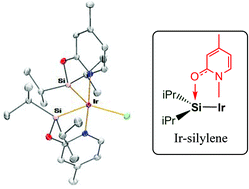
Iridium(III) complexes of the general formula [Ir(X)(κ2-NSiiPr2)2] (NSiiPr2 = (4-methyl-pyridine-2-yloxy)diisopropylsilyl; X = Cl, 3; CF3SO3, 5; CF3CO2, 6) have been prepared and fully characterized, including X-ray diffraction studies and theoretical calculations. The presence of isopropyl substituents at the silicon atom favours the monomeric structure found in complexes 3 and 5. The short Ir–Si bond distances (2.25–2.28 Å) indicate some degree of base-stabilized silylene character of the Ir–Si bond in 3, 5 and 6 assisted by the 2-pyridone moiety. However, the shortening of these Ir–Si bonds might be a consequence of the constrained 2-pyridone geometry, and consequently the silyl character of these bonds can not be excluded. A DFT theoretical study on the nature of the Ir–Si bonds has been performed for complex 3 as well as for four other iridium complexes finding representative examples of different bonding situations between Ir and Si atoms: silylene, base-assisted silylene (both with an anionic base and with a neutral base), and silyl bonds, using the topological properties of the electron charge density. The results of these studies show that the Ir–Si bonds in Ir–NSiiPr2 complexes can be considered as an intermediate between the base-stabilized silylene and silyl cases, and therefore they have been proposed as 2-pyridone-stabilized iridium silylene/silyl bonds.


 Please wait while we load your content...
Please wait while we load your content...