Synthesis and structural, electrochemical and photophysical studies of triferrocenyl-substituted 1,3,5-triphenylbenzene: a cyan-light emitting molecule showing aggregation-induced enhanced emission†
Abstract
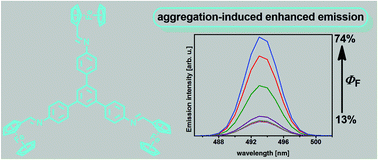
Triferrocenyl-substituted 1,3,5-triphenylbenzene was successfully synthesized in high yield. Single-crystal X-ray diffraction experiments revealed that the internal rotations of the ferrocenyl moieties are significantly restricted in the solid phase and that there are no significant π stacking interactions therein. The photoluminescence of the crystals is essentially the same as that of dilute chloroform solutions. However, studies of this cyan-light emitting substance in mixtures of chloroform and methanol revealed the aggregation-induced enhanced emission (AIEE) feature that boosts its fluorescence quantum yield from 13% up to 74%. We demonstrate the AIEE effect for a new class of easy-to-prepare aromatic molecules containing several metallocene units for the first time.


 Please wait while we load your content...
Please wait while we load your content...