Multicenter electron-sharing σ-bonding in the AgFe(CO)4− complex†
Abstract
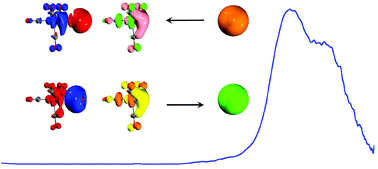
The heterodinuclear silver tetracarbonyl-iron anion was generated in the gas phase and studied by joint photoelectron velocity map imaging spectroscopy and quantum chemical calculations. The AgFe(CO)4− anion is characterized to be an 18-electron complex with the silver atom covalently bonded to the anionic tetracarbonyl-iron, an isolobal analogue of the methyl radical. The bonding analyses using a range of state-of-the-art quantum chemistry methods revealed a peculiar decentralized bonding situation, where the silver atom is covalently bonded to both the iron center and the vicinal carbon atoms in the form of an electron-sharing σ bond.


 Please wait while we load your content...
Please wait while we load your content...