Conductivity, structure, and thermodynamics of Y2Ti2O7–Y3NbO7 solid solutions
Abstract
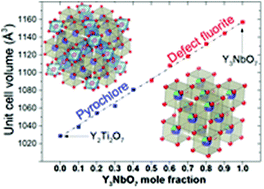
The defect fluorite yttrium niobate Y3NbO7 and pyrochlore yttrium titanate Y2Ti2O7 solid solutions have been synthesized via a solid state synthesis route. The resulting stoichiometry of the oxides is Y2+xTi2−2xNbxO7, where x = 0 to x = 1. All of the samples were single-phase; however, for those with a predominant fluorite phase, a small amount of additional pyrochlore phase was detected. The volume of the solid solution unit cells linearly increases with increase in yttrium niobate content. The water uptake increases with (x) and the protonic defect concentration reaches almost 4.5 × 10−3 mol mol−1 at 300 °C. The calculated enthalpy of formation from oxides suggests strong stability for all of the compositions, with the values of enthalpy ranging from −84.6 to −114.3 kJ mol−1. The total conductivity does not have a visible dependence on Y3NbO7 content. For each compound, the total conductivity is higher in wet air. Interestingly, for samples where x < 0.5, the ratio of conductivity in hydrogen to air increases with increasing temperature, while for x > 0.5, the trend is the opposite.


 Please wait while we load your content...
Please wait while we load your content...