Spontaneous conversions of glutamine, histidine and arginine into α-hydroxycarboxylates with NH4VO3 or V2O5†
Abstract
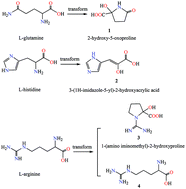
Glutamine gets transformed to 2-hydroxy-5-oxoproline with NH4VO3 in a neutral solution as a product of 2,2′-bipyridine oxidovanadium(V) 2-hydroxy-5-oxoproline [VV2O3(hop)2(bpy)2]·7H2O [1, H2hop = 2-hydroxy-5-oxoproline] with yields of 65.6%. Similarly, histidine and arginine are converted into the corresponding α-hydroxycarboxylates as 2,2′-bipyridine oxidovanadium(IV) 3-(1H-imidazolyl-5-yl)-2-hydroxyacrylate [VIV2O2(imha)2(bpy)2]·bpy [2, H2imha = 3-(1H-imidazolyl-5-yl)-2-hydroxyacrylic acid] and guanidinium oxidovanadium(V) 1-(aminoiminomethyl)-2-hydroxyproline (CN3H6)[VVO2(Haimhp)2]·2H2O [3, H2aimhp = 1-(aminoiminomethyl)-2-hydroxyproline] with V2O5 in low yields respectively, where an aggregate of oxidovanadium(V) arginine (H2arg)n(VVO3)n·½nH2O (4, Harg = arginine) has been isolated preferentially in an initial experiment for 3. α-Hydroxycarboxylates chelate bidentately with vanadium via α-alkoxy and α-carboxy groups in 1–3, as observed from structural analyses. Their racemizations have been observed after the conversions. There is no coordination in 4 based on solid-state 13C NMR spectra, and only strong hydrogen bonds exist in the anion chains (VVO3)− and protonated arginines. 1 and 4 were fully characterized by elemental analysis, UV–vis, IR and solid-state 13C NMR spectroscopies, TG and X-ray structural analyses, and theoretical bond valence calculations (BVS). The conversions of glutamine, histidine and arginine occur spontaneously in a solution under ambient conditions.


 Please wait while we load your content...
Please wait while we load your content...