N-Heterocyclic carbene and cyclic (alkyl)(amino)carbene adducts of gallium hydrides, gallium chlorides and gallium hydrochlorides†
Abstract
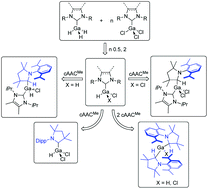
The synthesis and characterization of N-heterocyclic carbene (NHC) and cyclic (alkyl)(amino)carbene (cAAC) gallane and chlorogallane adducts of the type (NHC)·GaH3 (NHC = Me2ImMe1, iPr2Im 2, iPr2ImMe3 and Dipp2ImH4; Me2ImMe = 1,3,4,5-tetra-methyl-imidazolin-2-ylidene; R2Im = 1,3-di-organyl-imidazolin-2-ylidene; Dipp = 2,6-diisopropylphenyl; Dipp2ImH = 1,3-bis(2,6-diisopropylphenyl)-imidazolidin-2-ylidene), (NHC)·GaH2Cl (NHC = iPr2ImMe9, Dipp2Im 10 and Dipp2ImH11; iPr2ImMe = 1,3 diisopropyl-4,5-dimethyl-imidazolin-2-ylidene), (NHC)·GaHCl2 (NHC = iPr2ImMe12, Dipp2Im 13 and Dipp2ImH14) and (cAACMe)·GaHCl215 is reported. Compounds 1–3 and 9–11 are unstable in solution as heating to the boiling temperature of toluene (110 °C) leads to decomposition into elemental gallium and the corresponding dihydroaminal NHC–H2. The reaction of the mono-NHC adducts with a second equivalent of NHC also afforded decomposition and formation of NHC–H2, whereas the reaction of the NHC-stabilized gallanes with one equivalent cAACMe leads to an insertion of the cAACMe carbene carbon atom into the Ga–H bond. The synthesis and characterization of (NHC)·GaH2(cAACMeH) (NHC = Me2ImMe5, iPr2ImMe6 and Dipp2Im 7), the products of an oxidative addition of (NHC)·GaH3 to cAACMe, are presented. The adduct (Dipp2ImH)·GaH34 reacts with two equivalents of cAACMe under the release of Dipp2ImH and insertion of two cAACMe molecules into the Ga–H bond to give the bisalkylgallane (cAACMeH)2GaH 8. If one or two hydrogen atoms of the NHC gallane adducts are replaced with an electron-withdrawing chloride substituent, a selective insertion of cAACMe into the Ga–H bond occurs only for the adducts of sterically less demanding NHCs such as iPr2ImMe. The reaction of cAACMe with (iPr2ImMe)·GaH2Cl 9 and (iPr2ImMe)·GaHCl212 afforded (iPr2ImMe)·GaHCl(cAACMeH) 16 and (iPr2ImMe)·GaCl2(cAACMeH) 17. The reaction of cAACMe with (NHC)·GaHCl2 and (NHC)·GaH2Cl (NHC = Dipp2Im, Dipp2ImH), adducts of the sterically more demanding NHCs, leads to an extrusion of the NHC to (cAACMe)·GaHCl215 and (cAACMeH)2GaCl 18.


 Please wait while we load your content...
Please wait while we load your content...