1,3,4-Azadiphospholides as building blocks for scorpionate and bidentate ligands in multinuclear complexes†
Abstract
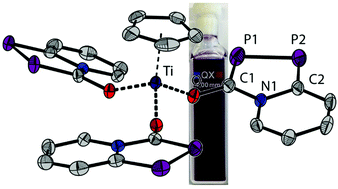
Annulated oxy-substituted 1,3,4-azadiphospholides such as the anion in Na[1] are readily accessible phosphorus heterocycles made from the phosphaethynolate anion (OCP)− and 2-chloropyridines. The sodium salt Na[1] reacts with oxophilic element halides such as OPCl3, PhSiCl3, PhBCl2 and CpTiCl3 at room temperature to form exclusively the oxygen bound tris-substituted compounds E(1)3 (with E = OP, PhSi, PhB− or CpTi). Six equivalents of Na[1] with group four metal chlorides MCl4 (M = Ti, Zr, Hf) form cleanly the hexa-substituted dianions (Na2[M(1)6]) which are isolated in excellent yields. The titanium complexes are deeply coloured species due to ligand to metal charge transfer (LMCT) excitations. In all complexes, the phosphorus atoms of the azadiphosphole moieties are able to coordinate to a soft metal center as shown in their reactions with [Mo(CO)3Mes], yielding complexes in which the Mo(CO)3 binds in a fac manner. Functionalization of the oxy group with amino phosphanes allows isolation of tridentate ligands, which have been used as synthons for macrocyclic molybdenum carbonyl complexes.


 Please wait while we load your content...
Please wait while we load your content...