Novel insights into the metal binding ability of ZinT periplasmic protein from Escherichia coli and Salmonella enterica†
Abstract
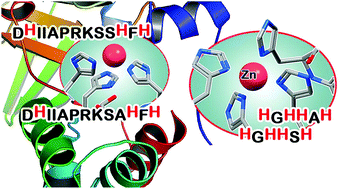
The ZinT mediated Zn(II) uptake is one of the major differences in the metabolism of human and bacterial cells that can be challenged when looking for possible highly selective metal-based therapeutics. ZinT is a 216-amino acid periplasmic protein expressed by Gram-negative bacteria, which shuttles Zn(II) ions to the ZnuABC transporter under zinc-limiting conditions. The suggested metal-binding sites of ZinT correspond to a domain containing three highly conserved histidine residues (His 167, 176 and 178) and to the N-terminal histidine-rich loop HGHHXH (residues 24–29). The coordination chemistry of the ZinT complexes with Zn(II) and Cu(II) has been investigated. The present work is focused on the protected peptides Ac-24HGHHSH29-NH2 and Ac-166DHIIAPRKSSHFH178-NH2 as models for the putative metal binding sites of ZinT from Escherichia coli (EcZinT), and Ac-24HGHHAH29-NH2 and Ac-166DHIIAPRKSAHFH178-NH2 from the ZinT protein expressed by Salmonella enterica sv. Typhimurium (SeZinT). The investigated peptides are able to form stable mono-nuclear complexes where the histidine residues represent the principal metal anchoring sites. The ZnuA (a periplasmic component of the ZnuABC transporter) metal binding site exhibits higher affinity for Zn(II) than ZinT, suggesting that the interaction of the two proteins through the formation of a binary complex may involve the metal transfer from ZinT to ZnuA. In contrast, this would not occur in Cu(II), since the ZinT complexes are more stable. Furthermore, at acidic pH, where the antimicrobial peptide calcitermin is biologically active, it also binds the metal ions with higher affinity than ZinT, representing a possible efficient competitor and antagonist of ZinT in the host human organism.


 Please wait while we load your content...
Please wait while we load your content...