Solvent-triggered stereoselectivity of α,α-cyclopropanation of amino acids in the Ni(ii) chiral coordination environment†
Abstract
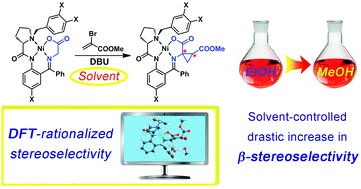
Experimental and DFT investigation of the α,α-cyclopropanation of amino acids via nucleophilic addition of the deprotonated glycine Ni(II)-Schiff-base complex, containing the (S)-proline derivatives as an auxiliary chiral moiety, to alkyl α-bromoacrylates was performed. It was demonstrated that the predominant configuration of the newly formed α-stereocenter is (S), regardless of the solvent used but the smart choice of solvent allows high diastereoselectivity at the removed β-stereocenter to be obtained, which commonly is rather rare. DFT analysis of the reaction path provides a rationale for the stereochemical outcome observed. The cyclopropanated complexes exhibit stereodependent redox activity, thus supporting that this is a general phenomenon inherent to this class of Ni Schiff-base derivatives, accounting for the influence of the peripheral groups in the metal coordination environment on the relative impact of different parts of the molecule in the frontier orbitals via conformational changes.


 Please wait while we load your content...
Please wait while we load your content...