Solid-state 1D → 3D transformation of polynitrile-based coordination polymers by dehydration reaction†
Abstract
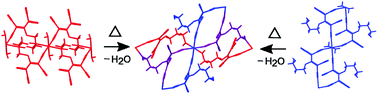
In crystal structures of two chain coordination polymers [M(tcnopr3OH)2(H2O)2] (M = NiII and CoII; tcnopr3OH− = [(NC)2CC(O(CH2)3OH)C(CN)2]−) based on a N,O or N,N′-bridge polynitrile ligand, the parallel chains are connected via, respectively, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H–O and O–H⋯O hydrogen bonds between uncoordinated functional groups of the ligand and coordinated water molecules. Upon heating, both solids undergo dehydration accompanied by degradation of their single crystals. Powder X-ray diffraction showed that non-isostructural triclinic single crystals transformed to isostructural monoclinic compounds. The solid-state reaction yielded 3D coordination polymers [M(tcnopr3OH)2] (M = NiII and CoII) based on a N,N′,O-connected tcnopr3OH−. Although previously tens of complexes based on tcnopr3OH and similar anions were synthesized and X-ray characterized, none of these contain a tridentate polynitrile ligand. Thus, this study provides evidence that solid-state reactions allow obtaining novel coordination modes of polynitrile ligands. The possible pathways for the transformation of H-bonded networks to 3D coordination polymers are discussed on the basis of the topological approach. Applicability of the topological approach to predict possible networks of solid-state reaction products based on the crystal structures of initial compounds is demonstrated.
N⋯H–O and O–H⋯O hydrogen bonds between uncoordinated functional groups of the ligand and coordinated water molecules. Upon heating, both solids undergo dehydration accompanied by degradation of their single crystals. Powder X-ray diffraction showed that non-isostructural triclinic single crystals transformed to isostructural monoclinic compounds. The solid-state reaction yielded 3D coordination polymers [M(tcnopr3OH)2] (M = NiII and CoII) based on a N,N′,O-connected tcnopr3OH−. Although previously tens of complexes based on tcnopr3OH and similar anions were synthesized and X-ray characterized, none of these contain a tridentate polynitrile ligand. Thus, this study provides evidence that solid-state reactions allow obtaining novel coordination modes of polynitrile ligands. The possible pathways for the transformation of H-bonded networks to 3D coordination polymers are discussed on the basis of the topological approach. Applicability of the topological approach to predict possible networks of solid-state reaction products based on the crystal structures of initial compounds is demonstrated.


 Please wait while we load your content...
Please wait while we load your content...