Reactive metallocene cations as sensitive indicators of gas-phase oxygen and water†
Abstract
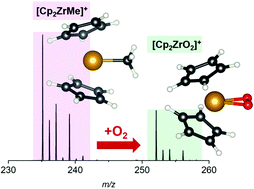
Analysis of highly reactive compounds at very low concentration in solution using electrospray ionization mass spectrometry requires the use of exhaustively purified solvents. It has generally been assumed that desolvation gas purity needs to be similarly high, and so most chemists working in this space have relied upon high purity gas. However, the increasing competitiveness of nitrogen generators, which provide gas purity levels that vary inversely with flow rate, prompted an investigation of the effect of gas-phase oxygen on the speciation of ions. The most reactive species studied, the reduced titanium complex [Cp2Ti(NCMe)2]+[ZnCl3]− and the olefin polymerization pre-catalyst [Cp2Zr(μ-Me)2AlMe2]+[B(C6F5)4]−, only exhibited detectable oxidation when they were rendered coordinatively unsaturated through in-source fragmentation. Computational chemistry allowed us to find the most plausible pathways for the observed chemistry in the absence of observed intermediates. The results provide insight into the gas-phase oxidation or hydrolysis of these reactive species.


 Please wait while we load your content...
Please wait while we load your content...