Product inhibition in nucleophilic aromatic substitution through DPPPent-supported π-arene catalysis†
Abstract
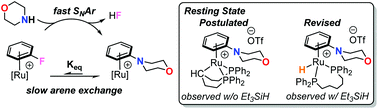
Nucleophilic aromatic substitution (SNAr) of fluorobenzene by morpholine at a bis(diphenylphosphino)pentane-supported ruthenim complex is investigated as a model system for π-arene catalysis through the synthesis and full characterization of proposed intermediates. The SNAr step proceeds quickly at room temperature, however the product N-phenylmorpholine binds tightly to the ruthenium ion. In the case examined, the thermodynamics of arene binding favor product N-phenylmorpholine over fluorobenzene binding by a factor of 2000, corresponding to significant product inhibition. Observations of the catalyst resting state support this hypothesis and demonstrate an additive-controlled role for a previously-proposed ligand cyclometalation.


 Please wait while we load your content...
Please wait while we load your content...