Peptoid-based siderophore mimics as dinuclear Fe3+ chelators†
Abstract
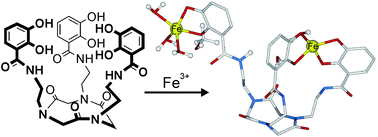
A practical synthesis of preorganized tripodal enterobactin/corynebactin-type ligands (consisting of a C3-symmetric macrocyclic peptoid core, three catecholamide coordinating units, and C2, C4, and C6 spacers) is reported. The formation of complexes with Fe3+ was investigated by spectrophotometric (UV-Vis) and spectrometric (ESI, negative ionization mode) methods and corroborated by theoretical (DFT) calculations. Preliminary studies revealed the intricate interplay between the conformational chirality of cyclic trimeric peptoids and metal coordination geometry of mononuclear species similar to that of natural catechol-based siderophores. Experimental results demonstrated the unexpected formation of unique dinuclear Fe3+ complexes.


 Please wait while we load your content...
Please wait while we load your content...