Antimicrobial properties of half-sandwich Ir(iii) cyclopentadienyl complexes with pyridylbenzimidazole ligands†
Abstract
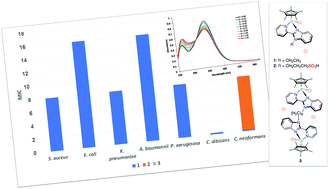
Reaction between 2-(2′-pyridyl)benzimidazole derivatives and [{IrCl(η5-C5Me5)}2(μ-Cl)2] afforded mono- and binuclear “piano-stool” Ir(III) compounds of type [IrnCln(η5-C5Me5)n(L)]Cln (n = 1, L = LET (1) and LSO3H (2); n = 2, L = LBN (3)), which were fully characterized, including the X-ray crystallographic analysis of 1. While the free ligands and compound 3 exhibited no toxicity to the tested microbes, compound 1 was highly potent against bacteria (MIC = 12.9–25.8 nM) and fungi (MIC < 0.40 nM). However, complex 1 induced damage to non-malignant cell lines (human embryonic kidney (HEK293), CC50 = 0.995 μg mL−1) and human RBCs (HC10 = 10.9 μg mL−1 and HC50 > 32 μg mL−1). Interestingly, complex 2, bearing the benzimidazole ligand with an alkylated sulfonate side chain (LSO3H), was selectively potent against C. neoformans with MIC value of 11.2 nM and was non-toxic to HEK293. According to DNA binding studies, compounds 1–3 could be considered as moderate metallo intercalators with a binding constant of 5.0 × 104–1.0 × 105 M−1. Alternatively, evidence was obtained, from ESI-MS measurements, for the non-covalent mode of binding of 2 to hen egg white lysozyme, while compounds 1 and 3 decomposed during the interaction with that protein. This may be attributed to the electrostatic and H-bonding interactions between the polar sulfonate group and charged protein side-chains.


 Please wait while we load your content...
Please wait while we load your content...