Cytotoxic and apoptotic effects of ternary silver(i) complexes bearing 2-formylpyridine thiosemicarbazones and 1,10-phenanthroline†
Abstract
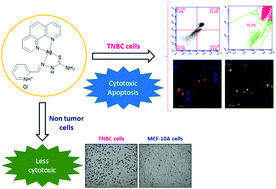
New silver(I) compounds containing 2-formylpyridine-N(4)-R-thiosemicarbazones and 1,10-phenanthroline (phen) were synthesized and characterized by spectroscopic techniques (IR and NMR), elemental analysis, ESI-MS and molar conductance measurements. In these complexes, both phen and thiosemicarbazone ligands are coordinated in a chelating bidentate fashion. Compounds 1–3 not only showed good in vitro antiproliferative activity against human lung (A549) and breast tumor cells (MDA-MB-231 and MCF-7), with IC50 values ranging from 1.49 to 20.90 μM, but were also demonstrated to be less toxic towards human breast non-tumor cells (MCF-10A). Cellular uptake studies indicated that compounds 1–3 were taken up by the MDA-MB-231 cells in 6 hours. Cell death assays in the MDA-MB-231 cells were conducted with compound 1 aiming to evaluate its effects on cell morphology, induction of apoptosis, the cell cycle, reactive oxygen species (ROS) formation and mitochondrial membrane potential (Δψm). Compound 1 caused morphological changes, such as cell shrinkage and rounding, increased the sub-G1 phase population, and induced apoptotic cell death, ROS formation and loss of mitochondrial membrane potential (Δψm). DNA binding results revealed that 1 interacted with the ct-DNA minor groove. Complexes 1–3 also exhibited good in vitro activity against M. tuberculosis H37Rv, with MIC values ranging from 3.37 to 4.65 μM.


 Please wait while we load your content...
Please wait while we load your content...