Synthesis and structural studies of copper(ii) complex with N2S2 based N-substituted pendant phosphonic acid arms†
Abstract
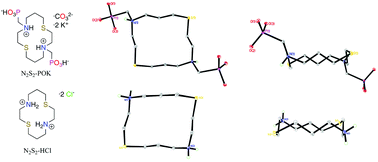
The synthesis of a heteromacrocyclic bifunctional chelator with phosphonic acid pendent arms is presented along copper(II) complexation. Ligand N2S2-POH featuring N,N′-bis-substituted phosphonate pendent arms was isolated in respectable yields, characterized, and chelated to copper(II). Implementation of both Moedritzer–Irani and Kabachnik–Fields conditions using aza–thia macrocycle 1,8-dithia-4,11-diazacyclotetradecane afforded 1,8-dithia-4,11-diazacyclotetradecane-4,11-diyl-bis-(methylene)-bis-(phosphonic acid) (N2S2-POH). Kinetic NMR studies provided four acid dissociation constants with respect to hydronium ion concentration. Benesi–Hildebrand binding experiment provided a conditional formation constant of 2.8 × 104 M−1. Heteromacrocycle N2S2-POH readily formed an encapsulated copper(II) chelate at room temperature, which was examined through EPR analysis.


 Please wait while we load your content...
Please wait while we load your content...