Reactivity of boraguanidinato germylenes toward carbonyl compounds and isocyanides: C–O, C–F and C–N bond activation†
Abstract
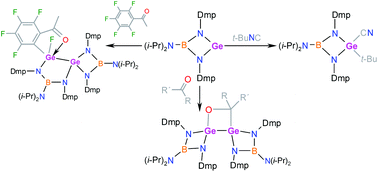
The reactions of two equivalents of germylene [(i-Pr)2NB(N-2,6-Me2C6H3)2]Ge (1) with carbonyl compounds RC(O)R′ resulted in carbonyl functionality activation and the formation of 4-(R,R′)-1,2-digerma-3-oxa-cyclobutanes (R/R′ = Ph/CF3 (2) or C6F5/H (3)). Surprisingly, the analogous reaction of 1 with C6F5C(O)Me led to the insertion of the germanium atom into the C–F bond of the perfluorophenyl group, thus producing a spiro compound (4) with a germanium atom sharing 1,2-digerma-3,5-diaza-4-bora-cyclopentane and 1-germa-2,4-diaza-3-boracyclobutane rings. Furthermore, the reaction of 1 with 2e− donors was investigated. In the case of 4-dimethylaminopyridine (DMAP), an expected complex [(i-Pr)2NB(N-2,6-Me2C6H3)2]Ge(DMAP) (5) was isolated, but using t-BuNC resulted in the formation of germanium(IV) cyanide [(i-Pr)2NB(N-2,6-Me2C6H3)2]Ge(CN)(t-Bu) (6) as a result of C–N bond activation in the starting isocyanide. In contrast, mixing other isocyanides RNC (R = Cy or Ad) with 1 in solution led only to an equilibrium between the starting compounds and most probably the corresponding complexes [(i-Pr)2NB(N-2,6-Me2C6H3)2]Ge(CNR) (R = Cy (7a) or Ad (8a)) based on NMR studies. From these equilibrium mixtures, fortuitously, single crystals of digerma-spiro-complexes (7 and 8) containing two germanium atoms (one of them coordinated to a particular isocyanide) were obtained and structurally authenticated by the X-ray diffraction technique.


 Please wait while we load your content...
Please wait while we load your content...