Protein interactions of dirhodium tetraacetate: a structural study†
Abstract
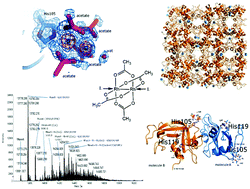
The interactions between the cytotoxic paddlewheel dirhodium complex [Rh2(μ-O2CCH3)4] and the model protein bovine pancreatic ribonuclease (RNase A) were investigated by high-resolution mass spectrometry and X-ray crystallography. The results indicate that [Rh2(μ-O2CCH3)4] extensively reacts with RNase A. The metal compound binds the protein via coordination of the imidazole ring of a His side chain to one of its axial sites, while the dirhodium center and the acetato ligands remain unmodified. Data provide valuable information for the design of artificial dirhodium-containing metalloenzymes.


 Please wait while we load your content...
Please wait while we load your content...