Structure, conductivity and magnetism of orthorhombic and fluorite polymorphs in MoO3–Ln2O3 (Ln = Gd, Dy, Ho) systems†
Abstract
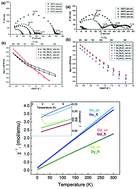
Phase-pure orthorhombic compositions at a Ln/Mo ratio ∼ 5.2–5.7 (Ln = Gd, Dy, Ho) have been obtained for the first time by prolonged (40–160 h) heat treatment of mechanically activated 5Ln2O3 + 2MoO3 (Ln = Gd, Dy, Ho) oxide mixtures at 1200 °C. Although the starting Ln : Mo ratio was 5 : 1 (Ln10Mo2O21 (Ln = Dy, Ho)), it changed slightly in the final product due to the volatility of molybdenum oxide at 1200 °C (40–160 h) (ICP-MS analysis). Brief high-temperature firing (1600 °C, 3 h) of 5Ln2O3 + 2MoO3 (Ln = Gd, Dy, Ho) oxide mixtures leads to the formation of phase-pure fluorites with compositions close to Ln10Mo2O21 (Ln = Gd, Dy, Ho). Gd10Mo2O21 molybdate seems to undergo an order–disorder (orthorhombic–fluorite) phase transition in the range of 1200–1600 °C. For the first time, using the neutron diffraction method, it was shown that low-temperature phases with a Ln/Mo ratio ∼ 5.2–5.7 (Ln = Gd, Dy, Ho) have an orthorhombic structure rather than a tetragonal structure. Proton contribution to the total conductivity of Ln10Mo2O21 (Ln = Gd, Dy, Ho) fluorites and gadolinium and dysprosium orthorhombic phases in a wet atmosphere was observed for the first time. In both orthorhombic and fluorite phases, the total conductivity in wet air decreases with decreasing lanthanide ionic radii. In a wide temperature range, the compounds under study exhibit paramagnetic behaviour. However, the orthorhombic phases of Dy and Ho compounds reach the antiferromagnetic state at 2.4 K and 2.6 K, respectively.


 Please wait while we load your content...
Please wait while we load your content...