Influence of initiating groups on phosphino-phenolate nickel catalyzed ethylene (co)polymerization†
Abstract
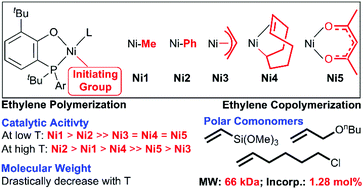
In transition-metal catalyst structures, both the ligand structure and the initiating group are crucial components for olefin polymerization. Compared to numerous studies on tuning the electronic and steric effects of ligands, there is no report on the comprehensive investigation of initiating groups. In this contribution, five different initiating groups including “NiMe”, “NiPh”, “Ni(allyl)”, “Ni(COD)”, and “Ni(acac)/AlEt2Cl” were designed and installed into sterically bulky phosphino-phenolate nickel complexes Ni1–Ni5, respectively, which were further tested for ethylene (co)polymerization. In ethylene polymerization, the order of activity was Ni1-PPh3 (NiMe) > Ni2 (NiPh) ≫ Ni3 (Ni(allyl)) = Ni4 (Ni(COD)) = Ni5 (Ni(acac)) at low temperature conditions (30 °C) with Ni1 being the most active group (850 kg mol−1 h−1). By comparison, at high temperatures (50 °C–90 °C), the activity followed the trend of Ni2 > Ni1-PPh3 > Ni4 ≫ Ni5 > Ni3 with Ni2 exhibiting the highest activity of 6290 kg mol−1 h−1. These results indicated that the choice of initiating groups was important in the polymerization reaction. In addition, Ni1-pyr and Ni2 enabled the copolymerization of ethylene with polar comonomers such as vinyl trimethoxysilane, 6-chloro-1-hexene, and nbutyl allyl ether to give polar functionalized polyethylenes with incorporation of up to 1.28 mol% and high molecular weights (up to 66 kDa).


 Please wait while we load your content...
Please wait while we load your content...