Interaction of Np(v) with borate in alkaline, dilute-to-concentrated, NaCl and MgCl2 solutions
Abstract
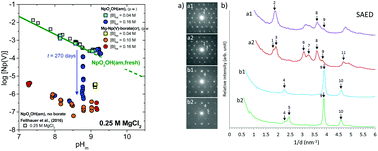
The interaction of Np(V) with borate was investigated in 0.1–5.0 M NaCl and 0.25–4.5 M MgCl2 solutions with 7.2 ≤ pHm ≤ 10.0 (pHm = –log[H+]) and 0.004 M ≤ [B]tot ≤ 0.16 M. Experiments were performed under an Ar-atmosphere at T = (22 ± 2) °C using a combination of under- and oversaturation solubility experiments, NIR spectroscopy, and extensive solid phase characterization. A bathochromic shift (≈5 nm) in the Np(V) band at λ = 980 nm indicates the formation of weak Np(V)–borate complexes under mildly alkaline pHm-conditions. The identification of an isosbestic point supports the formation of a single Np(V)–borate species in dilute MgCl2 systems, whereas a more complex aqueous speciation (eventually involving the formation of several Np(V)–borate species) is observed in concentrated MgCl2 solutions. The solubility of freshly prepared NpO2OH(am) remained largely unaltered in NaCl and MgCl2 solutions with [B]tot = 0.04 M within the timeframe of this study (t ≤ 300 days). At [B]tot = 0.16 M, a kinetically hindered but very significant drop in the solubility of Np(V) (3–4 log10-units, compared to borate-free systems) was observed in NaCl and dilute MgCl2 solutions with pHm ≤ 9. The drop in the solubility was accompanied by a clear change in the colour of the solid phase (from green to white-greyish). XRD and TEM analyses showed that the amorphous NpO2OH(am) “starting material” transformed into crystalline solid phases with similar XRD patterns in NaCl and MgCl2 systems. XPS, SEM–EDS and EXAFS further indicated that borate and Na/Mg participate stoichiometrically in the formation of such solid phases. Additional undersaturation solubility experiments using the newly formed Na–Np(V)–borate(cr) and Mg–Np(V)–borate(cr) compounds further confirmed the low solubility ([Np(V)]aq ≈ 10−6–10−7 M) of such solid phases in mildly alkaline pHm-conditions. The formation of these solid phases represents a previously unreported retention mechanism for the highly mobile Np(V) under boundary conditions (pHm, [B]tot, ionic strength) of relevance to certain repository concepts for nuclear waste disposal.


 Please wait while we load your content...
Please wait while we load your content...