Facile synthesis of a new Cu(ii) complex with an unsymmetrical ligand and its use as an O3 donor metalloligand in the synthesis of Cu(ii)–Mn(ii) complexes: structures, magnetic properties, and catalytic oxidase activities†
Abstract
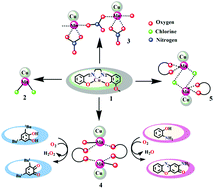
A new, facile Cu(II) template method has been employed for the unsymmetrical dicondensation of 1,2-ethylenediamine with salicylaldehyde and o-vanillin. The mononuclear complex, [CuL] (1), thus obtained, has been used as an O3 donor metalloligand for the synthesis of four new Cu(II)–Mn(II) complexes, [(CuL)MnCl2] (2), [(CuL)Mn(NO3)2(CH3OH)]n (3), {[(CuL)Mn(benz)(H2O)]2·(CuL)2(ClO4)2} (4) and [(CuL)Mn(benz)Cl]2 (5) (where benz = benzoate). Single-crystal structural analyses reveal that 2 is a dinuclear complex while complex 3 is polymeric with a repeating dinuclear [(CuL)Mn(NO3)2(CH3OH)] unit, linked via the nitrate ion. Both 4 and 5 are discrete tetranuclear complexes, where the dinuclear units [(CuL)Mn(benz)(H2O)] and [(CuL)Mn(benz)Cl] are connected by double benzoate and double chloride bridges, respectively. In complex 4, two monomeric [CuL] units are cocrystallized with the tetranuclear complex. An important difference in the structure of 4 from the other three complexes is that one solvent water molecule is coordinated to each Mn(II) ion, which makes complex 4 catalytically very active towards mimicking catecholase and phenoxazinone synthase-like oxidation reactions. The turnover numbers (kcat) for the aerial oxidation of 3,5-di-tert-butylcatechol and o-aminophenol are 399 h−1 and 230 h−1, respectively. The evidence of the intermediate species in the mass spectra indicates possible heterometallic cooperation where the Mn(II) center helps in substrate binding and Cu(II) participates in the oxidation reactions with molecular oxygen. Cyclic voltammetry measurements suggest the reduction of Cu(II) to Cu(I) during the catalytic process. Temperature-dependent dc molar magnetic susceptibility measurements reveal that complexes 2–5 are antiferromagnetically coupled with the exchange coupling constants (J) of J = −13.5 cm−1 and J = −13.5 cm−1 for 2 and 3, respectively, J1 = −12.6 cm−1 and J2 = −1.20 cm−1 for complex 4 and J1 = −13.24 cm−1 and J2 = 0.36 cm−1 for complex 5 as is expected from the Cu–O–Mn bridging angles.


 Please wait while we load your content...
Please wait while we load your content...